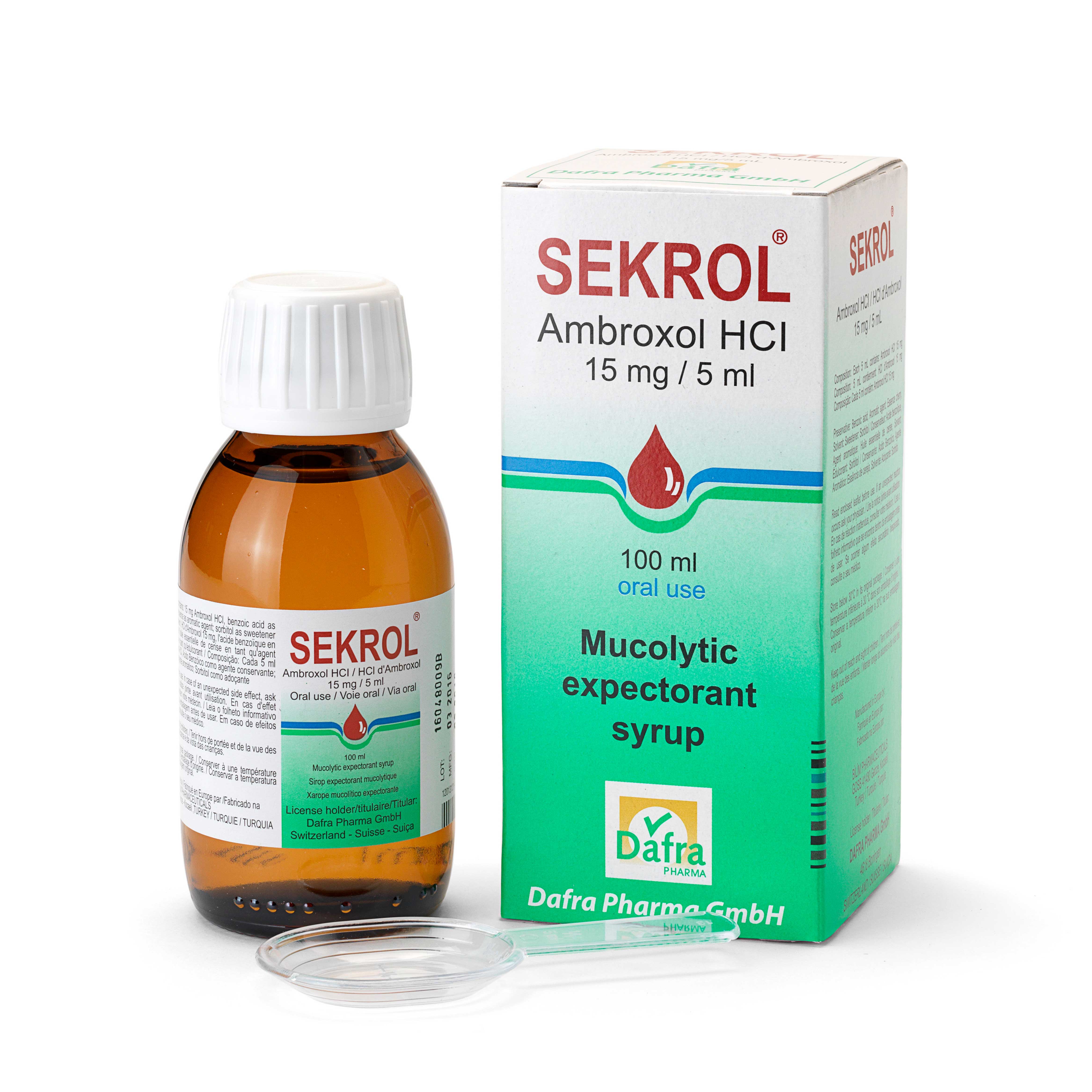
Active substance: ambroxol
Form: Syrup
Dosage:
– SEKROL pediatric: concentration of 15 mg ambroxol per 5 ml
– SEKROL adult: concentration of 30 mg ambroxol per 5 ml
Packaging:
– SEKROL pediatric: Bottle containing 100 ml of syrup and a 2.5-5ml measuring spoon
– SEKROL adult: Bottle containing 150 ml of syrup and a 2.5-5ml measuring spoon
Target: Pediatric form: children from 0 to 12 years
Adult form: adults and children from 12 years old
Sekrol is indicated as a mucolytic in the management of acute and chronic respiratory diseases that are characterised by viscid mucoid secretions (such as bronchitis, bronchiectasis, sinusitis).
Pediatric Form
For children under 2 years of age, the treating physician decides to use Sekrol 15mg/5ml individually. In the absence of medical advice, it is not recommended to give the medicine to children under 2 years of age.
For children over 12 years of age, Sekrol is available as a 30mg/5ml syrup.
Adult form (from 12 years old)
30 to 60 ml twice a day (5 to 10 ml twice a day)
Sekrol can be taken with or without food.
The concentration of the active substance in Sekrol adult is twice as high as in the paediatric form. 5 ml of the paediatric form is equivalent to 2.5 ml of the adult form.
Sekrol is suitable for diabetic patients.
Over-the-counter drug

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |