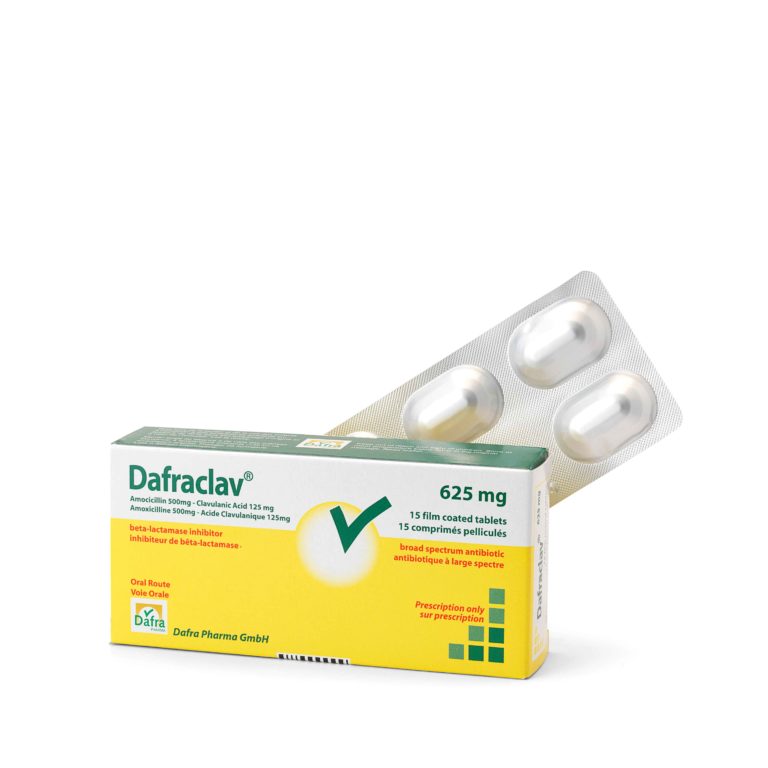
Active substance: amoxicillin – clavulanic acid
Form: film-coated tablet
Dosage per tablet: 500 mg amoxicillin + 125 mg clavulanic acid
Packaging: Box of 15 film-coated tablets
Target: adults and children over 6 years (>30kg)
For children under 30 kg Ticasse /Dafraclav is available as a suspension
Ticasse / Dafraclav 625mg is a bactericidal antibiotic
– Amoxicillin rapidly kills susceptible bacteria
– Clavulanic acid prevents the inactivation of amoxicillin by enzymes produced by bacteria.
Ticasse/Dafraclav is indicated for the treatment of the following infections
The usual dose for adults and children weighing 40 kg or more:
For children between 30 and 40 kg: 1 Ticasse/Dafraclav tablet every 12 hours.
Prescription drugs. Please contact your doctor.

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |