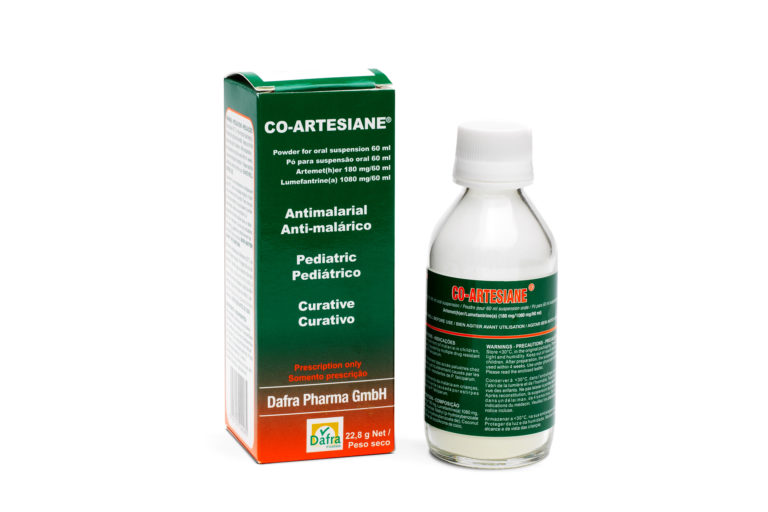
Active substance: artemether – lumefantrine
Form: Powder for oral suspension
Dosage:
After reconstitution Co-Artesiane contains a fixed dose combination of
– artemether: 3mg/ml
– lumefantrine: 18 mg/ml
Packaging:
Box containing a bottle for the preparation of 60 ml or 120 ml of drinkable suspension and a graduated measure Co-Artesiane 60 ml: Total content of 180mg arthemether and 1080mg lumefantrine
Co-Artesiane 120 ml: Total content of 360 mg arthemether and 2160mg lumefantrine
After reconstitution, the suspension has a yellow colour and a pleasant coconut taste appreciated by children
Target: children (> 5 kg)
Co-Artesiane is intended for paediatric use, but the product can also be used by adults.
Co‐Artesiane is indicated for the treatment of malaria in children
CO-ARTESIANE® suspension has been specially designed for children. The dosage depends on the severity of the case and the clinical condition of the patient: usually 4 mg artemether/kg body weight in combination with lumefantrine per day.
This dose will be taken for three consecutive days.
The daily dose is given as a single dose.
Simplified scheme: child’s weight (kg) x 1.5 = the amount (in ml) of Co-Artesiane per dose
Note: To avoid relapse, it is necessary to complete the full three-day course of treatment. If vomiting occurs within 30 minutes of taking the suspension, the full dose should be administered. If vomiting occurs within one hour of taking the suspension, half a dose should be administered.
Consult the scientific information leaflet for the dosage schedule.
Prescription drugs. Please contact your doctor.

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |