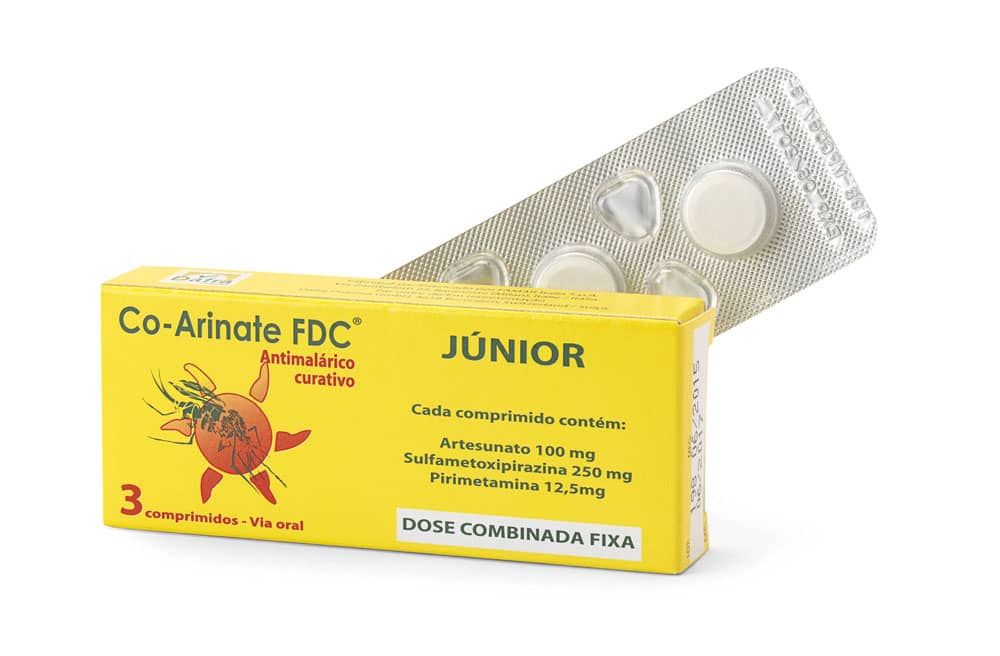
Active substance: artesunate – sulfamethoxypyrazine/pyrimethamine (SMP) as a fixed dose combination
Form : tablets
Dose per tablet :
Co-Arinate Enfant : 100 mg artesunate, 250 mg sulfamethoxypyrazine and 12,5 mg pyrimethamine
Co-Arinate Adulte : 200 mg artesunate, 500 mg sulfamethoxypyrazine and 25 mg pyrimethamine
Packaging : Box with 3 tablet blisters
Target :
Co-Arinate Junior : children from 9 kg to 35 kg (1 up to 13 y)
Co-Arinate Adult : adults and bigger children ( >35 kg) (14 y and older)
Further dose adaptations may be necessary for adults > 80 kg.
Co‐Arinate® is used as curative treatment for all forms of malaria, including severe malaria caused by multi drug‐resistant strains of Plasmodium falciparum.
The dose for this treatment is based on body weight at 4 mg / kg of Artesunate
combined with Sulfamethoxypyrazine and Pyrimethamine.
Three consecutive intakes of this dose must be given during 48 hours:
interval of 24 hours between doses which must be adhered to.
To avoid relapse, the full course of treatment should be completed.
If vomiting occurs within 30 minutes of taking the tablet, the full dose should be re-administered.
If vomiting occurs within one hour, half a dose should be re-administered.
Prescription drugs. Please contact your doctor.

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |