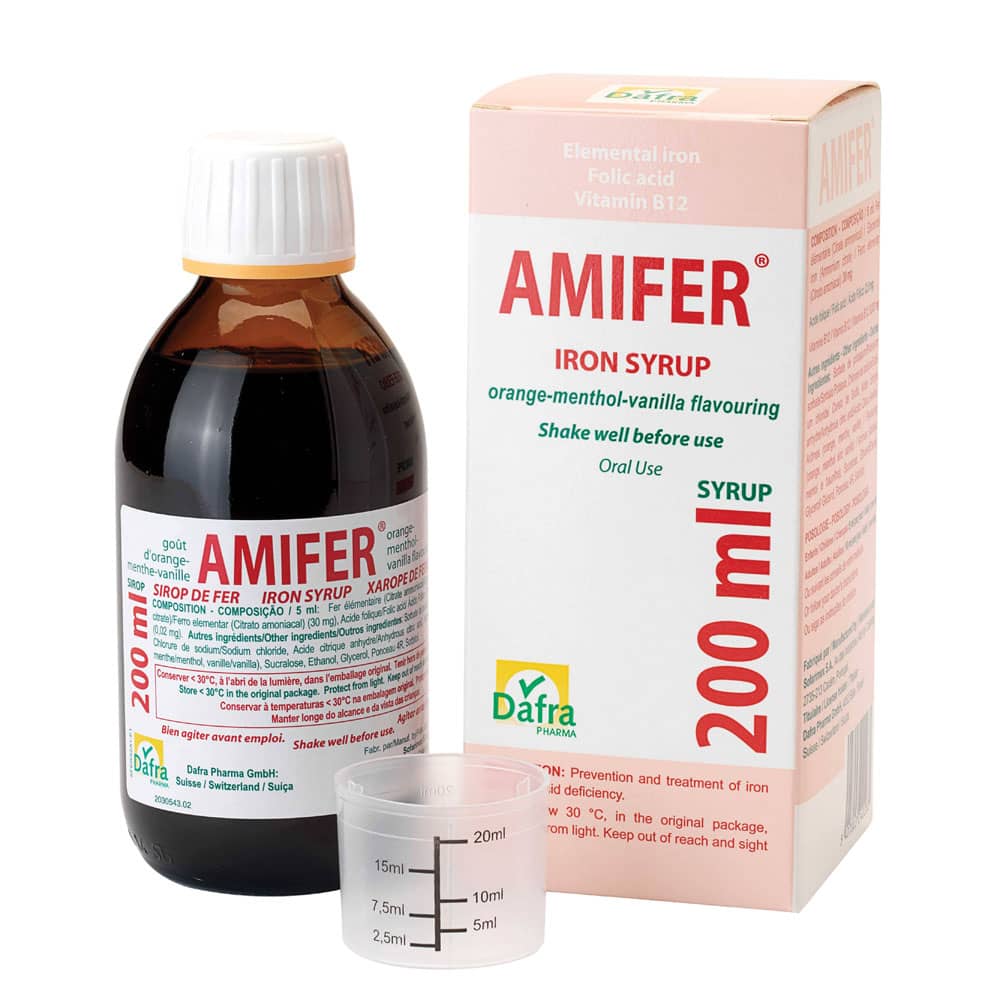
Active substance: Elemental iron – Folic acid (Vit B9) – Cyanocobalamin Vit B12
Form: Solution – Syrup
Dosage: per 5 ml
Packaging: Box with 200ml syrup and a plastic graduated measuring cup.
Target : adults and children (as from 1 y) – For infants Amifer® Junior Syrup is available in several countries
IRON SUPPLEMENTS (= to reduce deficiencies and replenish reserves)
Folic acid and Vit B12 supplementation
In case of anemia double the doses
Over-the-counter drug

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |